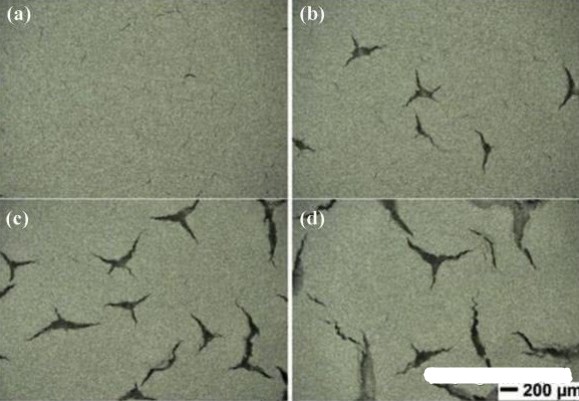
The detachment of electrode material in lithium batteries refers to the reduction of the contact area between electrode material and electrolyte, leading to a decrease in battery capacity and cycle life. Detachment typically occurs on the negative electrode material but may also occur on the positive electrode material. The main reasons for detachment are as follows:
1. Excessive Dynamic Stress: During the charge and discharge cycles of lithium batteries, the electrode material undergoes repetitive expansion and contraction processes. If this process is too intense, it can lead to the detachment of electrode material from the electrode sheet.
2. Oxidative Decomposition: During battery operation, the electrode material reacts with the electrolyte, forming new compounds. If the electrode material itself is unstable or if the electrolyte contains excessive impurities, it can result in oxidative decomposition of the electrode material, ultimately leading to detachment.
3. Insufficient Material Strength: Inadequate mechanical properties of the electrode material make it prone to fatigue and fracture. If the battery experiences mechanical impacts or vibrations during use, it can result in the detachment of electrode material.
4. Electrolyte Corrosion: Electrolytes containing highly corrosive substances can corrode the electrode material, eventually causing detachment.
To avoid the issue of electrode material detachment, it is necessary to use high-strength and stable materials, along with an appropriate amount of electrolyte. Additionally, controlling dynamic stress and preventing the battery from experiencing mechanical impacts and vibrations are essential measures.














